About the CLASS Lab
QC Procedures
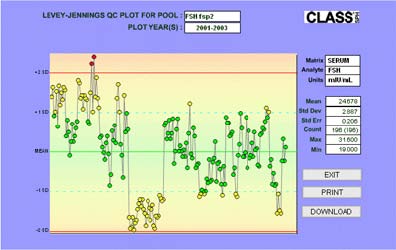
CLASS Laboratory serum quality control preparations include both commercially available controls and custom-prepared, in-house serum pools. For most assays, a commercially produced, multi-level quality control is purchased and used to monitor the low and high ranges of the assay. These are usually produced by the creator of the assay.
In addition to the commercial preparations, we maintain two pools (one from men; one from women) of sera which were screened for HIV and hepatitis virus. These pools were provided to us by the American Red Cross. Assays of these pools indicate a reasonable range of values for most of the hormones of interest. A single aliquot of each pool is quick-thawed daily with the samples, run and discarded after one day of use. Three to five controls chosen to cover the reporting range of individual assays are run in duplicate at the beginning and end of each assay. The Laboratory also has similar pools of urine and Saliva to cover all possible assays.
These QC preparations are intended to provide independent evidence of whether or not an observed change in values represents biological reality or change in methods or reagents as well as to provide evidence of the source of variability: menopausal transition, subject aging, site-handling, storage, or laboratory methods. Levy-Jennings plots are used to monitor drift in the QC samples.

